Who We Help

Cancer Researchers
We have experienced biostatisticians and data scientists who collaborate on study design, data analysis, and interpretation. We help your researchers develop and implement robust statistical methods, ensuring findings are accurate, reliable, and generalizable.
Pharmaceutical & Biotech Companies
Cancer Research And Biostatistics (CRAB) works directly with companies to design and conduct clinical trials, providing expertise in biostatistics, data analysis, and study design. This will help your company develop more effective and targeted therapies.

NIH-funded Projects
CRAB has contributed to NIH funded projects for 25+ years. In partnership with Fred Hutch Cancer Research Center, CRAB is also a part of the Statistics and Data Management Center for both the SWOG Cancer Research Network and Cancer Screening Research Network (CSRN).
How We Help

Biostatistics
We design and analyze of a wide variety of oncology clinical trials. Trials range from Phase I single or multi-institution industry sponsored to Phase III multi-institutional trials.
More About Biostatistic Services

Electronic Data Capture (EDC)
We securely capture, store, analyze and report research data in multiple EDC systems, including Medidata Rave and our own CRAB Nebula™ EDC.

Data Management
CRAB provides expert data collection, management and quality services, crucial to quality research and science.
Partner with CRAB
CRAB's expertise in biostatistics, data management, application development and DMC statistical and meeting management provides reliable and defensible results for your clinical trial.
Research
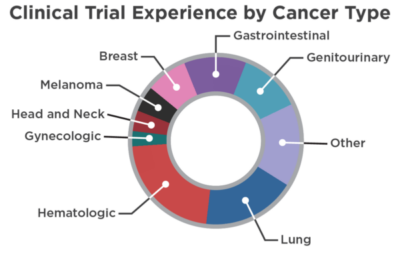
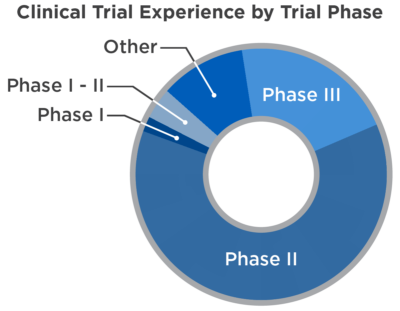
CRAB has supported more than 300 adult clinical trials. Many of the trials conducted through CRAB have been in large-scale Phase III trials. We have expertise across all trial phases including multi-institutional Phase I trials. The other studies (15%) are comprised of biological correlative studies, registries, post-marketing trials and outcomes research. CRAB statisticians have decades of experience in the design and analysis of a wide variety of oncology clinical trials.
Collaborations
CRAB achieves its mission through long-standing partnerships with both research collaborators and with for-profit organizations. The SWOG Statistics and Data Management Center (SDMC) is co-located at Fred Hutch and CRAB. SWOG is one of five U.S.-based National Cancer Institute-supported cooperative groups within the National Clinical Trials Network (NCTN). CRAB’s Clinical Research Services (CRS) division has a number of key partnerships with research consortia as well as pharmaceutical clients. Other important collaborations for CRAB are with Friends of Cancer Research (Friends) and the International Association For The Study Of Lung Cancer (IASLC).